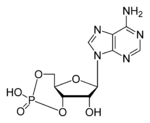
Cyclic adenosine monophosphate
| Cyclic adenosine monophosphate | |
|---|---|
 |
 |
| Identifiers | |
| CAS number | 60-92-4 |
| PubChem | 6076 |
| MeSH | Cyclic+AMP |
| Properties | |
| Molecular formula | C10H12N5O6P |
| Molar mass | 329.206 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Cyclic adenosine monophosphate (cAMP, cyclic AMP or 3'-5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is derived from adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.
Contents |
Synthesis and decomposition
cAMP is synthesised from ATP by adenylyl cyclase located on the inner side of the phospholipid bilayer. Adenylyl cyclase is activated by a range of signaling molecules through the activation of adenylyl cyclase stimulatory G (Gs)-coupled receptors and inhibited by agonists of adenylyl cyclase inhibitory G (Gi)-protein-coupled receptors. Liver adenylyl cyclase responds more strongly to glucagon, and muscle adenylyl cyclase responds more strongly to adrenaline.
cAMP decomposition into AMP is catalyzed by the enzyme phosphodiesterase.
Functions
cAMP is a second messenger, used for intracellular signal transduction, such as transferring the effects of hormones like glucagon and adrenaline, which cannot pass through the cell membrane. It is involved in the activation of protein kinases and regulates the effects of adrenaline and glucagon. It also regulates the passage of Ca2+ through ion channels.
In humans
.png)
cAMP and its associated kinases function in several biochemical processes, including the regulation of glycogen, sugar, and lipid metabolism.
In humans, cyclic AMP works by activating protein kinase A (PKA, cAMP-dependent protein kinase). PKA is normally inactive as a tetrameric holoenzyme, consisting of two catalytic and two regulatory units (C2R2), with the regulatory units blocking the catalytic centers of the catalytic units. Cyclic AMP binds to specific locations on the regulatory units of the protein kinase, and causes dissociation between the regulatory and catalytic subunits, thus activating the catalytic units and enabling them to phosphorylate substrate proteins.
The active subunits catalyze the transfer of phosphate from ATP to specific serine or threonine residues of protein substrates. The phosphorylated proteins may act directly on the cell's ion channels, or may become activated or inhibited enzymes. Protein kinase A can also phosphorylate specific proteins that bind to promoter regions of DNA, causing increased expression of specific genes. Not all protein kinases respond to cAMP. Several classes of protein kinases, including protein kinase C, are not cAMP-dependent.
Further effects mainly depend on cAMP-dependent protein kinase, which vary based on the type of cell.
Still, there are some minor PKA-independent functions of cAMP, e.g., activation of calcium channels, providing a minor pathway by which growth hormone-releasing hormone causes a release of growth hormone.[1]
In non-humans
Role of cAMP in bacteria
In bacteria, the level of cAMP varies depending on the medium used for growth. In particular, cAMP is low when glucose is the carbon source. This occurs through inhibition of the cAMP-producing enzyme, adenylyl cyclase, as a side-effect of glucose transport into the cell. The transcription factor cAMP receptor protein (CRP) also called CAP (catabolite gene activator protein) forms a complex with cAMP and thereby is activated to bind to DNA. CRP-cAMP increases expression of a large number of genes, including some encoding enzymes that can supply energy independent of glucose.
cAMP, for example, is involved in the positive regulation of the lac operon. In an environment of a low glucose concentration, cAMP accumulates and binds to the allosteric site on CRP (cAMP receptor protein), a transcription activator protein. The protein assumes its active shape and binds to a specific site upstream of the lac promoter, making it easier for RNA polymerase to bind to the adjacent promoter to start transcription of the lac operon, increasing the rate of lac operon transcription. With a high glucose concentration, the cAMP concentration decreases, and the CRP disengages from the lac operon.
Role of cAMP in some slime molds
In the species Dictyostelium discoideum, the chemotactic movement of cells is organized by periodic waves of cAMP that propagate through the cell. The waves are the result of a regulated production and secretion of extracellular cAMP and a spontaneous biological oscillator that initiates the waves at centers of territories.
Pathology
Role of cAMP in human carcinoma
Some research has suggested that a deregulation of cAMP pathways and an aberrant activation of cAMP-controlled genes is linked to the growth of some cancers.[2][3][4]
Role of cAMP in prefrontal cortex disorders
Recent research suggests that cAMP affects the function of higher-order thinking in the prefrontal cortex through its regulation of ion channels called hyperpolarization-activated cyclic nucleotide-gated channels (HCN). When cAMP stimulates the HCN, the channels open, closing the brain cell to communication and thus interfering with the function of the prefrontal cortex. This research, especially the degradation of higher cognitive function in ADHD when a person ages, is of interest to researchers studying the brain.[5]
See also
- Cyclic guanosine monophosphate (cGMP)
- 8-Bromoadenosine 3',5'-cyclic monophosphate (8-Br-cAMP)
- Acrasin specific to chemotactic use in Dictyostelium discoideum.
References
- ↑ GeneGlobe -> GHRH Signaling Retrieved on May 31, 2009
- ↑ American Association for Cancer Research (cAMP-responsive Genes and Tumor Progression)
- ↑ American Association for Cancer Research (cAMP Dysregulation and Melonoma)
- ↑ American Association for Cancer Research (cAMP-binding Proteins' Presence in Tumors)
- ↑ ScienceDaily ::Brain Networks Strengthened By Closing Ion Channels, Research Could Lead To ADHD Treatment
Additional images
|
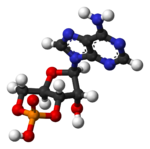
cAMP represented in three ways |
|
|||||||||||||||||||||||||||
